You must be signed in to read the rest of this article.
Registration on CDEWorld is free. You may also login to CDEWorld with your DentalAegis.com account.
Tooth whitening has been used for more than 150 years to improve the appearance of teeth.1 While the safety and efficacy of these old procedures were questionable, they were somewhat successful. They were also time consuming and involved the use of potentially dangerous chemicals. These protocols were primarily used for nonvital teeth. Almost a century ago, a pyrozone (ether peroxide) mouthwash proved effective in reducing caries and whitening teeth.2 Because these methods were performed in office, they often required multiple appointments. By the 1940s, hydrogen peroxide and ether were used to whiten vital teeth.3 In the late 1950s, nonvital teeth were treated with pyrozone and sodium perborate.
Klusmier is credited with introducing tray bleaching in the 1960s,4 but it was not until Haywood and Heymann published an article in 1989 that the procedure became well accepted.5 The introduction of carbamide peroxide greatly aided this acceptance because it was more stable chemically than hydrogen peroxide. Carbamide peroxide has a shelf life of 1 to 2 years compared with 1 to 2 months for hydrogen peroxide. Although other agents have been tried with varying degrees of success, the agent most commonly used in tray whitening techniques is carbamide peroxide combined with additives for flavor, color, desensitizing properties, and viscosity level.
This article is intended for the general practitioner or any practitioner who may speak to patients about whitening options. The article summarizes the current status of whitening techniques and discusses the following about tooth whitening:
• Mode of action
• Factors affecting the whitening process
• Safety and effects
• Types of techniques
• Special considerations for tetracycline stains
Mode of Action
The bleaching process allows the oxidizing agent to enter the enamel and dentin to produce a chemical action with discolored particles within the tooth structure. Hydrogen peroxide’s low molecular weight enables it to diffuse through the tooth’s organic matrix. The whitening agent’s exact action is not known, but it is theorized that free radicals attack organic molecules to achieve stability. This, in turn, releases other radicals to react with other unsaturated bonds to change the absorption energy of organic molecules. The result makes the molecules reflect less light so the tooth appears lighter. Early in the process, bleaching opens more highly pigmented carbon-ring compounds and converts them to lighter-colored chains (hydroxyl groups).6-8
Factors Affecting Speed of the Whitening Process
Several factors or conditions can alter the speed at which whitening occurs. While these effects are not precisely predictable, they should be considered before the whitening process is begun.
Surface Cleanliness
The target surfaces must be carefully cleaned to distinguish extrinsic from intrinsic stains. Removal of debris and surface film permits the whitening agent maximum contact with target surfaces. The whitening process may be delayed a few days if the prophylaxis causes any tooth or tissue sensitivity.
Peroxide Concentration
Higher concentrations produce a more rapid whitening effect.9 This effect is not linear (eg, 20% does not double the speed of 10%). Higher concentrations are more likely to result in increased tooth sensitivity. A 10% carbamide peroxide concentration is equal to approximately 3.4% concentration of hydrogen peroxide. Carbamide peroxide, while less concentrated than hydrogen peroxide, is much more stable with a shelf life of 1 to 2 years as opposed to hydrogen peroxide’s shelf life of only 1 to 2 months. A concentration of 10% carbamide peroxide degrades in the mouth to approximately 3.4% hydrogen peroxide and 6.5% urea.
Temperature of the Agent
The higher the agent’s temperature, the faster the oxygen release, which speeds the reaction.10 The chemical reaction rate can be doubled by an increase of 100°C.11 The speed of the color change may not be altered, and the rise in temperature may cause more pulpal sensitivity. To allow monitoring of any heat-provoked sensitivity, teeth should not be anesthetized during an in-office treatment that produces heat.12 The increased speed of the chemical reaction achieved by increasing the temperature may help explain the effect of some bleaching lights used with in-office procedures. The light’s benefit may be partially a product of its heat, not its specific light wave.
pH of the Agent
Agents with low pH can produce tooth-surface alterations. This can be avoided by using agents with a pH in the range of 7. The urea released during carbamide peroxide breakdown raises the pH above 8 for several hours. Hydrogen peroxide has an optimal range of 9.5 pH to 10.8 pH. A pH of 10.8 results in a whitening rate 50% faster than does 9.5 pH.13,14
Time
Results of the whitening process are directly related to the time the agent is in contact with the teeth. The longer the duration, the greater the degree of whitening is. However, extended periods may increase the likelihood of sensitivity.14,15
Safety and Effects
Many patients worry bleaching will harm their teeth or soft tissues or produce a systemic problem. Tooth whitening, whether through an in-office procedure or at-home delivery system, is very safe when the proper protocols are implemented.16 It is important to explain this to patients to let them weigh the benefits and minimal risks.
Systemic Effects
Soft Tissue
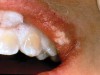
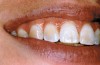
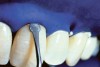
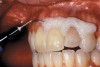
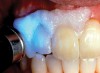
The whitening agents are available in various concentrations ranging from 10% carbamide peroxide (equal to 3.4% hydrogen peroxide) to 38% hydrogen peroxide. If the higher concentration agents contact the soft tissues, they can produce a chemical “burn” that turns the tissues temporarily white (Figure 1 and Figure 2). Although tissues quickly return to their normal color once they rehydrate, they may be mildly uncomfortable for a few hours. The patient may be distressed by the temporary appearance of the whitened soft tissues. A rubber dam or other protective barrier is mandatory to effectively seal off the tissues (Figure 3). In-office systems usually supply a light-cured resin in a syringe to apply around the cervical areas to confine the agent to the teeth (Figure 4 and Figure 5). In addition, flexible spreaders should be placed in the mouth to prevent the cheeks or lips from contacting the whitening agent.
Hard Tissue
Tooth sensitivity can occur in at least 50% of patients. Marson and Sensi reported as many as 63% experienced sensitivity.17 Consequently, patients should be warned of this. It would seem logical to assume that patients with certain conditions, including erosion, gingival shrinkage, leaking restorations, caries lesions, abrasion/attrition, and large pulps, would be particularly prone to developing significant sensitivity. However, research has not demonstrated that these conditions are dependable predictors of sensitivity.18-20
For many patients, sensitivity is not a significant problem. Symptoms may begin on the second or third day of tray whitening, starting with an awareness of a “tingling” sensation and/or slight cold sensitivity. Often, this sensitivity diminishes without any significant discomfort. If the patient experiences increasing sensitivity, he or she should cease bleaching for 1 to 2 days. Persistent sensitivity, especially if severe, can be treated with 5% potassium nitrate with fluoride incorporated into a gel similar to bleaching gel. Potassium nitrate has a numbing or calming effect on nerve transmission.21,22
Fluoride acts as a tubule blocker to limit the fluid flow to the pulp.23-25 It is placed into the bleaching tray in the same way as the bleaching agent.26
Generally, the patient notices some decrease in discomfort within a few hours. Bleaching-provoked tooth sensitivity is transitory, eventually disappearing even without treatment once the bleaching is completed. Sensitivity is thought to be caused by dentinal dehydration.27 Application of hydrating agents (eg, VivaSens®, Ivoclar Vivadent, www.ivoclarvivadent.us; Seal & Protect®, DENTSPLY Caulk, www.dentsply.com) prior to bleaching may reduce or prevent this dentin dehydration. Use of amorphous calcium phosphate has also been shown to be effective in preventing or eliminating dentin hypersensitivity both during and after bleaching treatment. It is deposited on the tooth surface and then mimics, to some extent, sclerotic dentin.28,29
Tooth sensitivity is difficult to predict. Patients with large pulps, loss of enamel, or carious lesions can be especially susceptible to sensitivity.30 However, studies have not indicated that tooth conditions, carious lesions, sex, or age is a predictor of sensitivity.30 The significant predictor appears to be pre-existing tooth sensitivity. These patients are the most likely to develop any discomfort. They can receive effective treatment with the potassium nitrate/fluoride gel or with amorphous calcium phosphate (MI Paste™, GC America, www.gcamerica.com) prior to beginning treatment or with bleaching gel interspersed throughout treatment.
Whitening and Existing Restorations
A decrease in surface microhardness of both microhybrid and nano-resin composites has been noted after exposure to Crest Night Effects® (Procter and Gamble, www.pg.com), Colgate Simply White Clear Whitening Gel (Colgate-Palmolive Co, www.colgate.com), and Opalescence® Quick (Ultradent, www.ultradent.com).31 Polishing restorations prior to and after exposure to bleaching agents is recommended.32 Often, patients with existing anterior restorations desire whitening their remaining teeth. They should be reassured that whitening agents will not injure restorative materials or alter their color. Teeth color will lighten, so existing restorations will appear darker. It may become necessary to replace restorations to match the new tooth shade. Teeth with facial veneers can be lightened somewhat if the underlying tooth is too dark. The agent is placed into the tray on the lingual side of the tooth for several days to a few weeks. Patients should be informed that the change will be viewed through the veneers so improvements will likely not be dramatic.
Whitening and Future Restorations
Multiple studies have shown the effects on bonding to tooth structure recently exposed to bleaching agents with a significant decrease in polymerization of adhesives applied to teeth immediately after bleaching. A 14-day delay before bonding achieved significant improvement in polymerization.33,34 Consistently, enamel bond strengths exhibit significantly lower bond strength when bonding was done immediately following bleaching.35
Types of Techniques
Various methods of tooth whitening procedures achieve some degree of success. These methods include tray bleaching, in-office direct application, placement of whitening strips, application of paint-on materials, and whitening toothpaste and mouthwash. Although the specific procedures or techniques differ, all whitening techniques rely on placement of a concentration of hydrogen peroxide or a hydrogen peroxide-releasing solution on the tooth and holding it against the surface for a specified period. Clinical studies have examined all these methods and demonstrated varying degrees of success. The specific techniques and predicted success are discussed in this article.
In Office
Currently, in-office procedures use a relatively high concentration of hydrogen peroxide ranging from 25% to 38% in a gel solution. The gel helps confine the material to the target areas and avoid contact with soft tissues. With such high concentrations, isolation of the teeth from the soft tissues becomes critical, as previously described. If the agent contacts soft tissues, affected areas should be noted, immediately rinsed with water to begin rehydrating the tissues, and then covered with an ointment such as Orabase® B (Colgate-Palmolive) to decrease discomfort and hasten return to normal color. The patient should be informed that the whitened condition of affected tissue is temporary and soft tissues should return to normal coloration in approximately 30 mins without residual effects. Most patients experience little postoperative discomfort.
Concentrations as high as 38% hydrogen peroxide produce fast results; however, the ultimate effect is not greater than with at-home tray bleaching with 10% carbamide peroxide. Although the original in-office bleaching agents were applied without any other aids, some companies now advocate procedures involving specially designed lights to enhance the effects. These lights are advertised to significantly speed the whitening process. The bleaching agent is applied to the target teeth, and then the teeth are exposed to the light for 15 to 20 mins. This procedure is repeated two to three times during the appointment. Limited evidence concerning the actual benefits of the lights is available. However, a recent study indicated that bleaching lights appear effective at speeding the whitening process for some patients.36 More research is needed to confirm these results.
In-office procedures have several potential advantages: whitening occurs more rapidly, they do not require patient compliance, and they facilitate focusing on specific teeth if extra applications are needed to match other teeth. This may be the treatment of choice for patients unable or unwilling to undergo the slower at-home procedures and may provide more patient compliance than is required for tray bleaching. However, the cost is greater because of the chair-time required and single treatment sessions may have limited results.37
Manufacturers have advertised dramatic in-office bleaching results, claiming that the whitening effect can be as dramatic as an eight- to 10-shade improvement. While that may be possible, the change should not be measured immediately after the bleaching appointment is completed. Isolation of the teeth to protect the soft tissues results in tooth dehydration. Dehydration causes teeth to appear whiter.38 Teeth take only 30 mins or less to dehydrate. However, teeth may require 1 hour to regain moisture after the normal intraoral environment has been re-established. Therefore, shade change comparisons made immediately after an in-office bleaching procedure are misleading. A better comparison is made 1 to 2 weeks postbleaching.
The duration that the agent remains on the teeth depends on its strength and the susceptibility of the teeth to develop sensitivity. Systems generally call for coating the teeth for 20 to 30 mins, rinsing away the agent, and then reapplying for another 20 to 30 mins. After each application period, the patient is asked if tooth sensitivity is occurring. If so, the procedure is discontinued until a subsequent appointment. Generally, three applications per appointment can be accomplished without significant tooth sensitivity during or after the procedure.
Manufacturers generally supply “follow-up” whitening agents to continue the whitening action initiated by the in-office procedure.39 These follow-up agents generally contain carbamide peroxide in a gel. It is either applied by the patient into a custom tray or already loaded in a manufacturer-supplied tray. The agent’s strength ranges from a relatively mild 10% carbamide peroxide to a much stronger 30% or even to a 10% hydrogen peroxide. The intention is to continue the whitening action for the next several days to achieve the desired final results.
A recent publication from the State University of New York compares an in-office bleaching technique with and without a light compared with an at-home bleaching kit.40 In this study, it was found that using an at-home kit with a relatively strong 32% carbamide peroxide for 3 mins daily produced a result of nine shades lighter after 4 weeks. Using the same kits, another group wore the trays for 15 mins a day, which resulted in a 12-shade difference in the same time frame.40
At Home
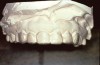
Generally, at-home treatment (tray bleaching) has been the most popular dentist-supervised tooth whitening method. It is generally less expensive, and the technique lends itself to an extended whitening regimen if tooth discoloration persists. The technique is relatively simple and easily accomplished. The only special equipment required is a vacuum-forming unit to create a well-fitting whitening tray designed specifically for the individual patient. The tray should be made on an accurate cast of the patient’s dentition. The cast should be trimmed to minimal thickness and with the palate or tongue space removed from the cast. This trimming promotes a better vacuum around the cast to create a better-fitting tray.
The tray should be formed from a soft plasticized sheet of clear material, such as ethyl vinyl acetate, that is approximately 0.035 inches thick. This material is easy to adapt and soft enough to fit comfortably. Although instructions for tray fabrication may recommend that the tray be trimmed short of the gingival margins to avoid contact of the agent with the tissues, research has not indicated that 10% to 15% carbamide peroxide irritates soft tissues. Tray borders must be well adapted and smooth to prevent irritation of the lips and cheeks and/or to keep the tongue from rubbing against rough edges (Figure 6 and Figure 7). The ethyl vinyl acetate tray material can be softened when heated with a “brush” flame and smoothed using finger pressure. In addition, there seems to be no significant advantage to creating a reservoir space for extra gel on the tray’s facial surface despite some manufacturers’ instructions. While it appears logical to create space for extra bleaching agents for prolonged/increased action, no research confirms its effectiveness. One study found that the amount of gingival inflammation is increased when trays are fabricated with reservoirs compared with nonreservoir trays.41 The patient should practice placing the tray in the office to ensure that he or she can do it correctly. While seating the tray may seem simple, the patient may not understand how to do it the first time.
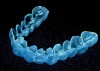
Figure 6 and Figure 7 Proper adaptation and trimming of a whitening tray on a maxillary cast. Tray showing both the buccal and lingual aspects scalloped.
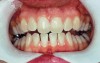
Use of a relatively low-strength agent such as 10% carbamide peroxide will require considerable time to achieve the desired effect. The key to success lies in holding the agent in intimate contact with the tooth surfaces for the necessary time.42 The custom tray loaded with whitening gel will achieve this if left in the mouth for the time required (Figure 8 and Figure 9). Some systems use a much stronger agent—25% to 38% carbamide peroxide or 10% or higher hydrogen peroxide. These more concentrated agents should be in place for only 30 mins. Recommended time to wear the tray varies depending on the agent’s strength.
Figure 8 and Figure 9 Prior to at-home whitening. Following approximately 10 days of at-home tray whitening.
Studies have shown that carbamide peroxide gel provides some bleaching effect even up to 6 hours after tray insertion.42 It is at maximal effectiveness for the first 2 hours before its strength begins to diminish. The best recommendation is to advise the patient to wear the tray during sleep. The patient should brush his or her teeth and then insert the tray before bedtime. This recommendation has two advantages: the time the agent contacts the teeth is maximized and the wearing time is during sleep when saliva flow is less. However, patients who do not tolerate wearing the tray during sleep can do the bleaching during the day. For the best benefits from the gel, the patient should wear the tray at least 2 hours.
The higher the concentration of peroxide, the more rapid the change.43,45 This faster change has disadvantages, however, because stronger agents increase the likelihood of tooth sensitivity.44,46 If the patient persists with the whitening process despite increasing sensitivity, significant discomfort may occur and take some time to resolve.
Over the Counter
With the growing popularity of tooth whitening in the last 15 years, the number of over-the-counter (OTC) products have proliferated. These products appear, in some cases, with strong advertising but have little research to prove their efficacy. They may use a stock tray system, a tray that can be softened and custom-formed to some extent, or a paint-on process. While the actual bleaching agent may be similar to the dentist-supplied-and-supervised agent, it is the method of placing and holding the agent on the teeth that determines the product’s efficacy. As previously stated, an important factor to successful whitening is the time the teeth are exposed to the whitening agent. The whitening effect of many OTC products, including toothpastes and mouthwashes, is questionable because of the limited period that they are in contact with the teeth.
Research has proven one OTC system is effective.47,48 Crest Whitestrips® (Procter and Gamble) has had about 15 years of success. This product involves placement of a strip resembling a Band-Aid over the anterior teeth for 30 mins twice daily. The system has some disadvantages, however. It may be difficult to adapt to misaligned teeth. The strip covers from canine to canine only in many mouths. The result is that the anterior teeth may lighten several shades but the premolars retain their original color. The patient may also wish to cover the premolars; however, this means using extra strips, adding to the cost.
Other systems may work; however, research has shown that results vary greatly depending on the agent employed and the means of applying and retaining it on the teeth. A paint-on system from Colgate has been shown to be effective but has the disadvantage of requiring the patient to carefully coat the target teeth and then keep the agent in place for approximately 30 mins. Some patients may find this difficult or tedious.49,50
Patients need to be warned that they may spend considerable money on some OTC products without achieving the results that are predictably obtained with a dentist-supervised-and-directed method.
Other products such as toothpaste are advertised as tooth whitening agents. While they may contain the same type of peroxide as the dentist-supplied agents, they do not remain on the teeth long enough to provoke the chemical process that eliminates the undesired coloration. Research does not show that “whitening” toothpastes are any more effective than regular toothpaste.51
Special Considerations for Tetracycline Trays
Some discolorations are highly persistent. Tetracycline discoloration can prove almost impossible to completely eliminate and difficult to lighten to an acceptable degree. Tetracycline medications are very effective antibiotics but can create a tooth esthetic problem. Treatment of an expectant mother during the third trimester or a child during tooth formation stages between 3 and 4 months of age and again at 7 to 8 years allows tetracycline to accumulate in the tooth buds.52,53 Presumably, treatment with tetracycline performed in expectant mothers in their first trimester should be safe; it is usually not done for precautionary reasons. If taken long term during early adulthood (eg, acne treatment), it can produce discoloration. This is especially true if ingested during secondary dentin formation, during growth periods, or following tooth trauma. The result is a distinctive and persistent discoloration with varying intensities of yellow, gray, blue, or brown. Its most characteristic overtone is a bluish gray.
Tetracycline-discolored teeth do eventually lighten from bleaching albeit much more slowly than teeth stained by other agents.54 In fact, whitening tetracycline-discolored teeth may take 2 to 12 months of daily treatment to achieve a satisfactory result. However, teeth usually retain grayish overtones. Teeth will generally lighten from the incisal toward the gingiva, so teeth heavily stained in the cervical area have the most limited prognosis.55
Vital tooth bleaching has provided a partial solution to these problems. Although tetracycline stains are persistent, they do eventually respond. Studies documenting treatment of tetracycline-stained vital teeth are limited; however, they demonstrate that stains can be minimized, if not completely eliminated. The improvement in coloration can be enough to allow use of more translucent and esthetic porcelain for veneers to complete the esthetic improvement. There is a persistent belief that tetracycline stains do not respond to either at-home or in-office “power” bleaching or even to a combination of the two. Patients may decide, therefore, to forego treatment.
The procedure for whitening these teeth may involve both in-office and at-home whitening procedures. The in-office procedure with 35% hydrogen peroxide serves as a “jump-start” to initiate the process. This is followed by at-home whitening, as previously discussed. The at-home treatment can be interspersed with occasional in-office “boosts” to the process. The patient must be informed that the incisal two thirds of the tooth will likely demonstrate the greatest color improvement. The gingival one third will be distinctly slower in improvement and may never completely lose a bluish or grayish tint. This is because the enamel is much thinner at the cervical area of the tooth so the underlying stained dentin remains.
Conclusion
Although manufacturers continue to package their products differently, especially OTC products, the basic ingredients remain constant. Whitening action is accomplished by release of hydrogen peroxide or carbamide peroxide that becomes hydrogen peroxide. Specific additives improve the taste, help confine the agent to the teeth, decrease likelihood of sensitivity, and/or prolong shelf life. In-office procedures are effective; however, one appointment is unlikely to be sufficient. Use of lights to enhance bleaching effects has not been fully proven effective at this point. OTC products vary widely in efficacy, primarily because of variations in methods of applying and retaining the bleaching agent to the teeth. Decades of whitening teeth have proven the process to be effective and safe if done according to accepted protocols.
References
1. How WS. Esthetic dentistry. Dent Cosmos. 1886;3:741-745.
2. Atkinson CB, Hints, queries and comments: pyrozone. Dent Cosmos. 1893;35:330-332.
3. Smith MS, McInnes JW. Further studies on methods of removing brown stains from mottled teeth. J Am Dent Assoc.1942;29:571.
4. Haywood VB, Drake M. Research on whitening teeth makes news. NC Dent Rev. 1990;7(2):9.
5. Haywood VB, Heyman HO. Nightguard vital bleaching. Quintessence Int. 1989;20(3):173-176.
6. Bowles WH, Thompson LR. Vital bleaching: the effect of heat and hydrogen peroxide on pulpal enzymes. J Endod. 1986;12:108-112.
7. Cooper JS, Bokmeyer TJ, Bowles WH. Penetration of the chamber by carbamide peroxide bleaching agents. J Endod. 1992;18(7):315-317.
8. Haywood VB. Current concepts: bleaching of vital teeth. Quintessence Int. 1997;28(6):424-425.
9. Matis BA, Wang Y, Jiang T, et al. Extended at-home bleaching of tetracycline-stained teeth with concentrations of carbamide peroxide. Quintessence Int. 2002;33(9):645-655.
10. Fasanaro TS Bleaching teeth: history, chemicals, and methods used for common tooth discolorations. J Esthet Dent. 1992;4(3):71-78.
11. Seale NS, McIntosh JE, Taylor AN. Pulpal reaction to bleaching of teeth in dogs. J Dent Res. 1981;60(5):948-953.
12. Goldstein RE, Garber DA. Complete Dental Bleaching. Chicago, IL: Quintessence; 1995:15,60.
13. Zaragoza VMT. Bleaching of vital teeth: technique. Estomodeo. 1984;9:7-30.
14. Frysh H, Bowles WH, Baker F, et al. Effect of pH on bleaching efficiency [abstract A2248]. J Dent Res. 1993:72(spec iss)384.
15. Haywood VB, Leonard RH, Dickinson GL. Efficacy of six months of nightguard vital bleaching of tetracycline-stained teeth. J Esthet Dent. 1997;9(1):13-19.
16. Li Y. Peroxide-containing tooth whiteners: an update. Compend Contin Educ Dent. 2000;28(suppl):S4-S9.
17. Marson FC, Sensi LG, Vieira LC, et al. Evaluation of in-office dental bleaching treatments with and without the use of light-activation sources. Oper Dent. 2008;33(1):15-22.
18. Nathanson D, Parra C. Bleaching vital teeth: a review and clinical study. Compend Contin Educ Dent. 1987;8:490-497.
19. Leonard RH Jr, Haywood VB, Phillips C. Risk factors for developing tooth sensitivity and gingival irritation associated with nightguard vital bleaching. Quintessence Int. 1997;28(8):527-534.
20. Leonard RH Jr, Bentley C, Eagle JC, et al. Nightguard vital bleaching: a long-term study on efficacy, shade retention, side effects, and patients’ perceptions. J Esthet Restor Dent. 2001;13(6):357-369.
21. Hodosh M. A superior desensitizer-potassium nitrate. J Am Dent Assoc. 1974;88:831-832.
22. Markowitz K. Tooth sensitivity: mechanisms and management. Compend Contin Educ Dent. 1993;14(8):1032-1046.
23. Gaffar A. Treating hypersensitivity with fluoride varnishes. Compend Contin Educ Dent. 1998;19(11):1088-1097.
24. Tam L. Effect of potassium nitrate and fluoride on carbamide peroxide bleaching. Quintessence Int. 2001;32(10):766-770.
25. Jerome CE. Acute care for unusual cases of dentinal hypersensitivity. Quintessence Int. 1995;26(10):715-716.
26. Haywood VB, Caughman WF, Frazier KB, et al. Tray delivery of potassium nitrate-fluoride to reduce bleaching sensitivity. Quintessence Int. 2001;32(2):105-109.
27. Betke H, Kahler E, Reitz A, et al. Influence of bleaching agents and desensitizing varnishes on the water content of dentin. Oper Dent. 2006;31(5):537-542.
28. Geiger S, Matalon S, Blasbalg J, et al. The clinical effect of amorphous calcium phosphate (ACP) on root surface sensitivity. Oper Dent. 2003;28(5):496-500.
29. Giniger M, MacDonald J, Ziemba S, et al. The clinical performance of professionally dispensed bleaching gel with added amorphous calcium phosphate. J Am Dent Assoc. 2005;136(3):383-392.
30. Goldstein RE. Bleaching teeth: new materials—new role. J Am Dent Assoc. 1987(spec no):44E-52E.
31. Okte Z, Villalta P, García-Godoy F, et al. Surface hardness of resin composites after staining and bleaching. Oper Dent. 2006;31(5):623-628.
32. Polydorou O, Hellwig E, Auschill TM. The effect of different bleaching agents on the surface texture of restorative materials. Oper Dent. 2006;31(4):473-480.
33. Breschi L, Cadenaro M, Antoniolli F, et al. Extent of polymerization of dental bonding systems on bleached enamel. Am J Dent. 2007;20(4):275-280.
34. Barghi N, Godwin JM. Reducing the adverse effect of bleaching on composite-enamel bond. J Esthet Dent. 1994;6(4):157-161.
35. Miyazaki M, Sato H, Sato T, et al. Effect of whitening agent application on enamel bonding strength of self-etching primer systems. Am J Dent. 2004;17(3):151-155.
36. Ontiveros JC, Paravina RD, Ward MT. Clinical evaluation of a chairside whitening lamp and bleaching efficacy [abstract 1081]. J Dent Res. 2008;(spec iss).
37. Goldstein C. In practice. Contemp Esthetics Restorative Pract. 2002;7:12-17.
38. Russell MD, Gulfraz M, Moss BW. In vivo measurement of colour changes in natural teeth. J Oral Rehabil. 2000;27(9):786-792.
39. Miller MB. Power bleaching—does it work or is it marketing hype? Prac Proced Aesthet Dent. 2002;14:636.
40. Kim-Pusateri S, Ciancio SG, Bessinger M, et al. Clinical comparison of bleaching effect of in-office to home bleaching [abstract 1080]. J Dent Res. 2009(spec iss).
41. Kirsten GA, Freire A, de Lima AA, et al. Effect of reservoirs on gingival inflammation after home dental bleaching. Quintessence Int. 2009;40(3):195-202.
42. Haywood VB. Nightguard vital bleaching: current information and research. Esthet Dent Update. 1990;1(2):7-12.
42. Matis BA. Tray whitening: what the evidence shows. Compend Contin Educ Dent. 2003;24(4A):354-362.
43. Leonard RH, Sharma A, Haywood VB. Use of different concentration of carbamide peroxide for bleaching teeth: an in vitro study. Quintessence Int. 1998;29(8):503-507.
44. Kihn PW, Barnes DM, Romberg E, et al. A clinical evaluation of 10 percent vs. 15 percent carbamide peroxide tooth-whitening agents. J Am Dent Assoc. 2000;131(10):1478-1484.
45. Matis BA, Mousa HN, Cochran MA, et al. Clinical evaluation of bleaching agents of different concentrations. Quintessence Int. 2000;31(5):303-310.
46. Matis BA, Cochran MA, Eckert G, et al. The efficacy and safety of a 10% carbamide peroxide bleaching gel. Quintessence Int. 1998;29(9):555-563.
47. Gerlach RW. Shifting paradigms in whitening; introduction of a novel system for vital tooth bleaching. Compend Contin Educ Dent. 2000;21(suppl):S4-S9.
48. Gerlach RW, Gibb RD, Sagel P. Initial color change and color retention with a hydrogen peroxide bleaching strip. Am J Dent.2002;15(1):3-7.
49. Slezak B, Santarpia P, Xu T, et al. Safety profile of a new liquid whitening gel. Compend Contin Educ Dent. 2002;23(11 suppl 1):4:4-11.
50. Gerlach RW. Shifting paradigms in whitening: introduction of a novel system for vital tooth bleaching. Compend Contin Educ Dent. 2000;21(suppl):S4-S9.
51. Slezak B, Santarpia P, Xu T, et al. Safety profile of a new liquid whitening gel. Compend Contin Educ Dent. 2002;23(11 suppl 1):4-11.
52. Moffitt JM, Cooley RO, Olsen NH, et al. Prediction of tetracycline-induced tooth discoloration. J Am Dent Assoc. 1974;88(3):547-552.
53. Mull MM The tetracyclines and the teeth. Dent Abstr. 1967;12:346-350.
54.Haywood VB, Leonard RH. Six- and 12-month color stability after 6-months bleaching tetracycline teeth [abstract 2891]. J Dent Res. 1996;75(spec iss);379.
55. Haywood VB, Leonard RH, Dickinson GL. Efficacy of six months of nightguard vital bleaching of tetracycline-stained teeth. J Esthet Dent. 1997;9(1);13-19.